Properties of Sand | Physical Properties of Sand | Chemical Properties of Sand

Table of Contents
Properties of Sand
It can be used as soon as it is obtained from the source. Contains binding content (5-20 per cent), water (5-8 per cent) and a significant amount of organic matter. The moisture content should be preserved for a long time.
Finishing on natural sand moulds is fine. It’s cheaper than most sands. It has less refractoriness. It is used for casting CI and non-ferrous metals. Natural sand moulds can be quickly repaired.
When combined with bentonite, the properties of sand are enhanced and have properties like Synthetic Sand.
1. Physical Properties of Sand
Here the different types of physical proprietors of sand are as follows with full detail.
- Grain Size of Sand
- Grain Shape of Sand
- Shape and Distribution of Sand Grains
- Size Distribution of Sand
a. Grain Size of Sand

The grain shape and scale of the moulding sand determine the overall surface area of the grains found in the unit mass. Total surface area is defined as a particular surface area. A particular surface provides a rough understanding of the quantity of binder required to coat grains of sand moulding.
Grain size and distribution affects many properties of sand, such as permeability, flowability, refractory properties, surface fineness and strength. The finer the grains of sand, the finer the entire sand. Fine grain sands offer a good surface finish but have poor permeability.
With the same content of clay, the environmental strength is greater in the context of fine sand than in the context of rough sands. Solid and evenly graded sand has high permeability, decent reactiveness as well as high flowability. Usually, foundry sand has grain sizes around 0.1 and 1.0 mm.
Fine-grained sands are being used to the importance of ensuring as well as small castings. Large grained sands are being used to produce large castings.
Also Read: What Is Sand Blasting | Concrete Sand-blasting Equipment | Key Benefits of Sand Blasting
Useful Article for You
- What Is a Highway Flyover
- What Is Grouting
- What Is a Pile Cap
- What Is a Bond Beam in Masonry
- What Is Sapwood
- What Is Crane
- What Is a Gable
- What Is Superelevation
- What Is Kerb
- What Is the Purpose of Washers
- What Is the Size of a Brick in Inches
- What Is Reinforced Masonry
- What Is Workability
- What Is Bond Breaker
- What Is Plasticizer in Concrete
- What Is Luminous Flux Vs Lumens
- What Is Caisson
- What Is an Undercoat
- What Is a Benchmark Surveying
- What Is Bracing in Construction
- What Is a Beam in Construction
- What Is the Standard Door Frame Size
- What Is a Spandrel Beam
- What Is a Fire Escape
- What Is a Weep Hole
- What Is Tie Beam
- What Is Fine Aggregate
- What Is Pony Wall
- What Is Flag Stone
- What Is Development Length
- What Is Cement Plaster
- What Is a Pitched Roof
- What Is a Slab in Construction
- What Is a Monolithic Slab
- What Is Linear Distance
- What Is Shovel
- What Is Lintel in Construction
- What Is a Concept Sketch
- What Is Mezzanine Floor
- What Is Man Sand
- What Is Plaster Made Out of
- What Is a Floating Slab
- What Is Falsework
- What Is Bituminous
- What Is a Spillway
- What Is Curb and Gutter
- What Is Dampness
- What Is Lap Length
- What Is the Full Form of Fsi
- What Is Door Frame
- What Is Plinth Protection
- What Is Traffic Rotary
- What Is Grade Slab
- What Is Rolling Margin of Steel
- What Is Modulus of Rupture
- What Is Fresh Concrete
- What Is Dpc in Construction
- What Is Earthen Dam
- What Is Plum Concrete
- What Is Shell Structure.
- What Is Lumber
- What Is the Strongest Foundation for a House
- What Is the Meaning of Soundness of Cement
- What Is Flyover Bridge
- What Is Under Reamed Pile
- What Is Weir
- What Is Inverted Beam
- What Are the Advantages of Levelling?
- What Is Sunk Slab
- What Is Brick Bat Coba
b. Grain Shape of Sand

The shape of the grain is defined in terms of angularity as well as sphericity. The grains of sand range from well rounded, subrounded, angular and also very angular. Inside each angularity unit, grains can be of a high, medium, or low sphericity. The angularity of sand is measured by visual inspection using a low-power microscope.
The best foundry sands include grains that have been rounded with medium to high sphericity, offering goof flowability and high-intensity permeability at low binder additions. More angular and lower spherical sands require higher binder additions, lower packing density and poorer flowability.
c. Shape and Distribution of Sand Grains

The texture, size and form of the sand grains are critical for controlling the consistency of the mould. Many of the mould aggregates are mixtures of fresh sand and recycled sand, which comprise not only reclaimed sand but also core sands.
In deciding the scale, shape and distribution of sand grains, it is necessary to note that the grain shape corresponds to the amount of sand surface area and that the distribution of grain size influences the permeability of the mould.
If the sand surface area increases, the volume of bonding content (usually clay and water) must increase if the sand is to be adequately bonded. As a result, a difference in surface area, possibly due to a change in sand form or the proportion of core sand being retrieved, would result in a related change in the amount of bond required.
Rounded grains have a low surface-area-to-volume ratio and are thus favoured for core processing because they need the least amount of binder. However, when recycled into the sand moulding system, their form can be disadvantageous if the molding system usually uses a high percentage of clay and water to allow quick, automatic moulding. This is because the rounded grains need less sand than the rest of the structure.
Angular sands have the highest surface area (with the exception of sands that crack quickly and contain a significant proportion of small grains and fine sands) and thus require more mulling, bonding and moisture. The angularity of sand rises with its use when the sand is broken by thermal or mechanical shocks.
The porosity of the mould regulates its permeability, i.e. the capacity of the mould to allow the gasses produced during the spilling to pass through all the mould. The maximum porosity is attributed to grains that are all about the same size.
As the size range widens, there are plenty more particles that are tiny enough just to occupy the gap between large grains. When grains break down by constant recycling, there seem to be smaller grains as well as the porosity of the mould reduces.
Furthermore, if the porosity of a mould is too high, the metal will penetrate sand grains and create a burn-in flaw. It is also important to balance the delivery of base sand and proceed to screen the sand and then use dust collectors throughout recycling to extract fines and to decide the correct bond addition.
d. Size Distribution of Sand
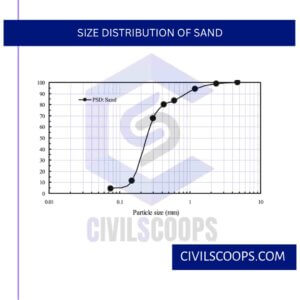
The size distribution of sand influences the consistency of the casting. Coarse grain sand allows the metal to enter the moulds and cores, giving the castings a weak surface finish.
Fine-grained soils produce a better surface finish but need a higher bonding content and low permeability can cause a gas defects in castings.
2. Chemical Properties of Sand
Here the different types of physical chemicals of sand are as follows with full detail.
- Soil Reaction (PH) of Sand
- Silicate Clay Presence in Sand
- Cation Exchange Capacity (CEC) in Sand
a. Soil Reaction (PH) of Sand

By reference, “PH” is a calculation of the concentration of active hydrogen ions (H+). It is an indicator of the acidity or alkalinity of the soil and is therefore defined as the “soil reaction.”
The pH varies from 0 to 14, for values under 7.0 acid as well as values above 7.0 alkaline. The pH value of 7 is known to be neutral, where H+ and OH-are equivalent, all at a concentration of 10-7 moles/liter. The pH of 4.0 is ten times more acidic than the pH of 5.0.
The most significant influence of ph in the soil is on ion solubility, and this, in turn, determines microbial growth including plant growth. The initial ph of 6.0 to 6.8 is suitable for many of these plants as it fits the optimal solubility of the most essential plant nutrients.
Few minor elements (e.g. iron) yet most toxic chemicals are much more soluble at reduced PH. This allows pH management essential for regulating the transport of toxic metals (and possible contaminated groundwater) in soil.
With construction and also an industrial perspective, it is important to decide the pH of the soil mass. The acidic, as well as basic essence of the soil, should be understood for growing healthy crops.
In the other side, in building work, strongly acidic soils would have a bearing on the road’s bitumen stability and detrimental effects on concrete strength. (The salinity of soils can also raise cost of maintenance)
Hydrogen, as well as aluminium, become dominant interchangeable cations in acid soils. The latter would be soluble within acidic conditions, and its reactivity with water (hydrolysis) creates hydrogen ions. Calcium, as well as magnesium, are simple cations; the relative sum of acidic cations increases as their concentrations increase.
Factors influencing soil pH include parent material, vegetation and atmosphere. Some rocks and sediments contain more acidic soils than others: quartz-rich sandstone is acidic; and limestone is alkaline. Some types of vegetation, especially conifers, produce organic acids that can contribute to lower soil pH values.
In wetlands such as the eastern US, soils begin to become more acidic with time, because runoff washes out essential cations and replaces them with hydrogen.
b. Silicate Clay Presence in Sand

The presence of silicate clay content influences the chemical properties of the soil mass. Clay particles have a wide surface area and are the best materials in the soil mass.
Clay particle increases the reactivity of the soil mass and influences the cohesion of the soil mass by forming compounds with foreign materials.
The assessment of the occurrence of silicate clay is necessary in order to determine the reactivity of the soil mass and its consistency with the mixtures and building materials used in concrete.
Useful Article for You
- How Wide Is a Cinder Block
- How Much Is a Coffered Ceiling
- How to Make Mortar
- How Long Does Hempcrete Last
- How to Use a Hand Sight Level
- How to Build a Lean to Roof
- How Are Tunnels Built
- How to Layout a Building
- How Wide Is a Car Parking Space
- How Do Shear Walls Work
- How to Measure Concrete Slump
- How to Use Washers with Screws
- How Dense Is Sand
- How High Is a Window from the Floor
- How Does a Beam Bridge Work
- How Do They Pour Concrete Under Water
- How Does a Sewer System Work
- How High Are Countertops
- How to Seal Brick Wall Interior
- How to Resurface Cement
- How to Use Portland Cement
- How Is Plaster Made
- How to Find Fineness Modulus
- How to Get Rid of Spray Paint Smell on Metal
- How Many Types of Slope Are There
- How Big Is a Stair Landing
- How to Get Paint Off Concrete Without Chemicals
- How to Fix Water Damaged Drywall
- How Much to Get Septic Pumped
- How to Cut a Nail or Screw
- How Long Does Wet Concrete Take to Dry
- How Is Varnish Made
- How Does Ejector Pump Work
- How Does Hydrometer Work
- How to Get Wet Blood Out of Carpet
- How to Build House on Slope
- How Thick Is Plaster Wall
- How Suspension Bridges Work
- How to Seal a Concrete Roof
- How Was Cement Invented
- How to Calculate Area of Steel
- How to Check Silt Content in Sand
- How a Building Is Constructed
- How Are Roads Classified in India
- How Many Types of Cement in India
- How to Find Contour Interval
- How to Stop Leakage from Ceiling
- How Hardness of Brick Is Tested
- How Many Types of Paint Brushes Are There
- How to Calculate Skirting Area
- How Many Types of Beam
- How to Make Road
- How Many Types of Chain in Surveying
- How to Find One Way and Two Way Slab
- How Many Types of Houses
- How to Find Steel Bar Weight
- How to Calculate Septic Tank Capacity in Liters
- How to Calculate the Bearing Capacity of Soil
- How Many Types of Bricks Are There
c. Cation Exchange Capacity (CEC) in Sand

Any plant nutrients and metals occur in the soil atmosphere as positively charged ions or “cations.” Among the most important soil cations are hydrogen (H+), aluminium (Al+3), calcium (Ca+2), magnesium (Mg+2) and potassium (K+). Many heavy metals also occur in the soil ecosystem as cations.
Clay and organic matter particles are primarily negatively charged (anions) and have the capacity to keep cations from being “leached” or washed away. Adsorbed cations are subject to substitution by other cations in a quick, reversible mechanism called cation exchange.
The Cation Exchange Capacity of the soil is indeed an estimate of the maximum of the negative value at a given weight of the sample, or the sum of cations that a given soil sample can carry in an interchangeable shape.
Cations escaping the exchange sites meet the soil particles where they’ll be absorbed by plants, interact with several other soil elements, or transported with runoff water.
The higher the quantity of clay as well as organic matter much higher the Cation Exchange Capacity, while various forms of clay minerals and organic matter may differ in Cation Exchange Capacity.
Cation exchange is an important process in soils for the preservation and delivery of plant nutrients and for the adsorption of pollutants. It plays an important function in soil wastewater treatment.
Sandy soils with low CEC are usually unsuitable for septic systems because they have no adsorptive capability and there is a potential for groundwater.
[su_box title=”FAQ” style=”default” box_color=”#333333″ title_color=”#FFFFFF” radius=”3″ class=”” id=””]
Properties of Sand
- Should be completely inert.
- Grains should be sharp, strong & angular.
- Should not contain any hygroscopic salts (i.e., CaCl2, MgCl2, etc.).
- Should not contain clay & silt; usually 3-4% clay & silt is ordinarily permitted for practical reasons.
- There should be no organic matter.
What Is Sand?
Sand is a granular material composed of finely divided mineral particles. Sand has various compositions but is defined by its grain size. Sand grains are smaller than gravel and coarser than silt.
Quality of Good Sand
Here are some of the common good quality sand properties: There should be a sharpness to the grains and a coarseness to them. In order to ensure that the sand is of high quality, it must be free of clay, organic matter, and be a proper sand. There should be no organic matter mixed with the sands.
Sand Physical Properties
As sand is one of the known type of soils, it shares the same properties that are used to categorize them. The most relevant properties for sand are: texture, structure, color, and consistency.
Chemical Properties of Sand
The main element of quartz sand is silicon dioxide (SiO2). The oxygen atoms form with the silicon atom in the centre a tetrahedral structure. Each oxygen atom simultaneously belongs to two silicon atoms. In this manner the tetrahedral structures have a cross-linked high-molecular weight.
Chemical Composition of Sand
Description of SiO2
The chemical compound silicon dioxide, also known as silica (from the Latin silex), is an oxide of silicon with a chemical formula of SiO2 and has been known for its hardness since antiquity. Silica is most commonly found in nature as sand or quartz, as well as in the cell walls of diatoms.
Chemical Composition of Silica Sand
Specifically, silica sand is made up of silicon dioxide (SiO2). The most common form of SiO2 is quartz – a chemically inert and relatively hard mineral. SiO2 grades at a 7 out of 10 on Mohs hardness scale, making it ideal for use as filtration media and abrasive blasting sands.
What Are the Properties of Sand?
- Particle Size: Sand particles range from 0.0625 mm to 2 mm in diameter.
- Color: Can vary widely, including white, brown, red, yellow, and black.
- Texture: Gritty and granular to the touch.
- Density: Typically between 1.6 to 2.7 grams per cubic centimeter.
Physical Properties of Sand
- 0.0625 mm to 2 mm in diameter.
- Various shades including white, brown, red, yellow, and black.
- Gritty and granular.
- 1.6 to 2.7 grams per cubic centimeter.
River Sand Chemical Composition
The primary chemical component of sand is silica, followed by smaller amounts of alumina, iron oxide, and calcium oxide. The chemical composition of sand is primarily SiO2, or silica. Silica, by definition, is a component of soil, rock, and other minerals.
[/su_box]
[su_note note_color=”#F2F2F2 ” text_color=”#333333″ radius=”3″ class=”” id=””]
Like this post? Share it with your friends!
Suggested Read –
- H-Beam vs I-Beam
- Water Damage in Bathroom
- What Is Lightweight Concrete
- What Is Recycled Concrete Aggregate
- Specific Gravity Test of Bitumen
[/su_note]
Originally posted 2023-11-07 11:38:54.
